
RegenPRP™, CELL EXTRACTS AND CELLULAR MATRIX® COMBINATIONS: 10 YEARS OF CLINICAL RESEARCH AND PRACTICE.
Pr Barbara HERSANT MD, PhD. | Department of Plastic, Reconstructive and Maxillofacial Surgery, Hôpital Henri Mondor, Créteil, France Article in French
Summary
Platelet Rich Plasma (RegenPRP™) has emerged as an innovative therapeutic modality in a variety of medical fields, offering promising prospects for wound healing, facial rejuvenation and even genital rejuvenation.
Our clinical research is resolutely committed to studying these applications, seeking to determine the efficacy and safety of RegenPRP™ in these varied clinical contexts.
- In the field of wound healing, RegenPRP™ is attracting growing interest due to its regenerative properties and potential to accelerate the healing process. Our research aims to deepen our understanding of how RegenPRP™ can optimize the healing of post-operative and traumatic wounds, thereby improving clinical outcomes for patients.
- Facial rejuvenation is an area where RegenPRP™ has become an increasingly popular option. By harnessing the cell regeneration capabilities of RegenPRP™ , our study examines how this therapy can be used safely and effectively to alleviate the signs of skin aging, offering a non- surgical alternative to traditional facial rejuvenation techniques.
- In a revolutionary move, our research is also exploring the possibilities of genital rejuvenation using RegenPRP™. This innovative approach aims to restore the function and appearance of the genitalia, offering a new means of treatment for patients suffering from sexual dysfunction and aesthetic problems in this region.
It should be noted that all our studies are conducted in compliance with the strictest ethical and regulatory standards. Approved by the French medical agency and supervised by an independent ethics committee, our prospective studies guarantee scientific rigor and participant safety.
Our clinical research on RegenPRP™ is opening up exciting new perspectives in the fields of wound healing, facial rejuvenation and genital rejuvenation. By combining a rigorous scientific approach with a commitment to medical innovation. We aim to improve clinical outcomes and patient quality of life with this promising therapy.
Keywords: RegenPRP™, Cellular Matrix®, combination of RegenPRP™ and hyaluronic acid, plastic surgery, skin grafting, vulvovaginal atrophy, keloid scars, mesenchymal stromal cells.
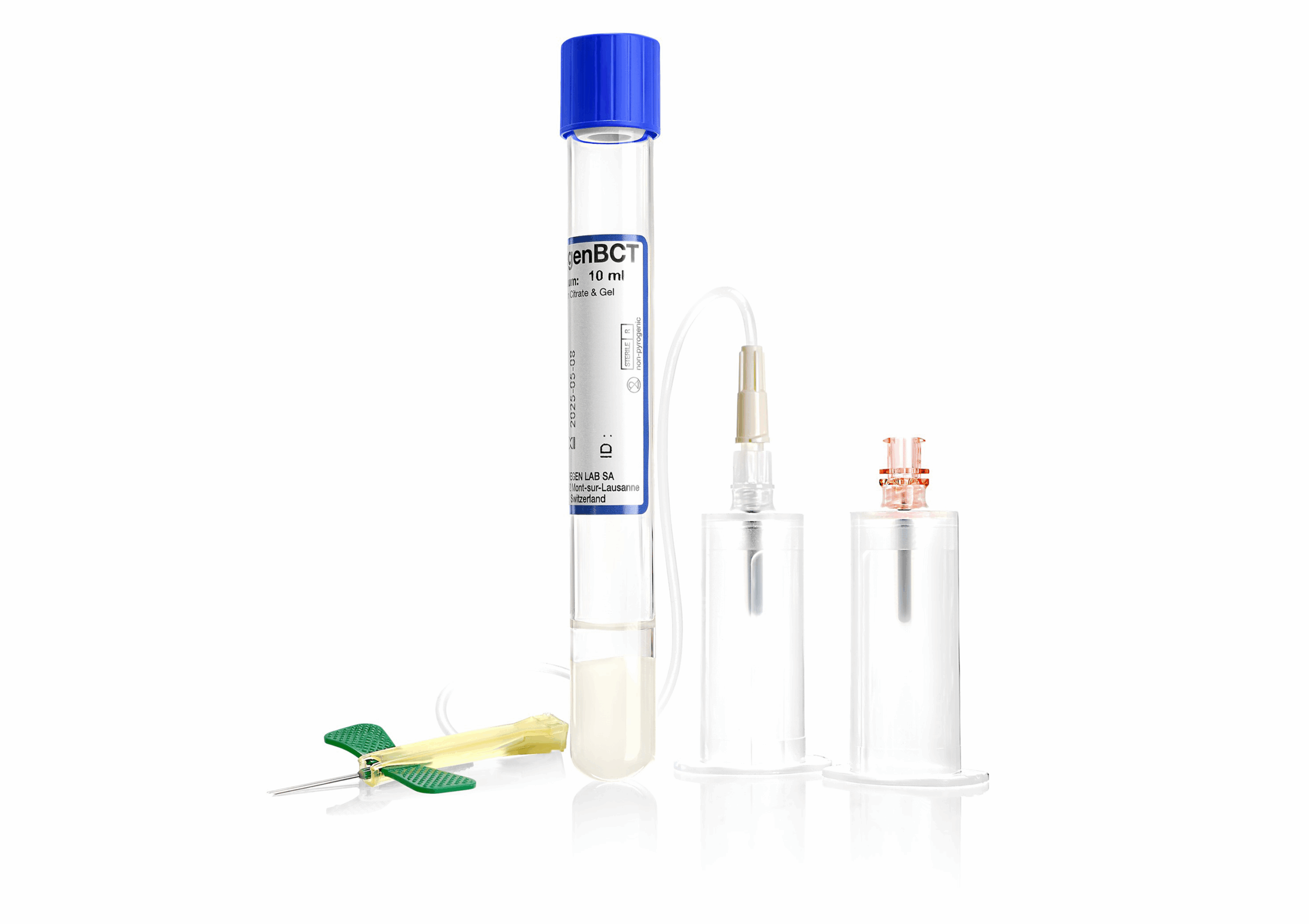
Figure 1. RegenBCT® (10 mL)
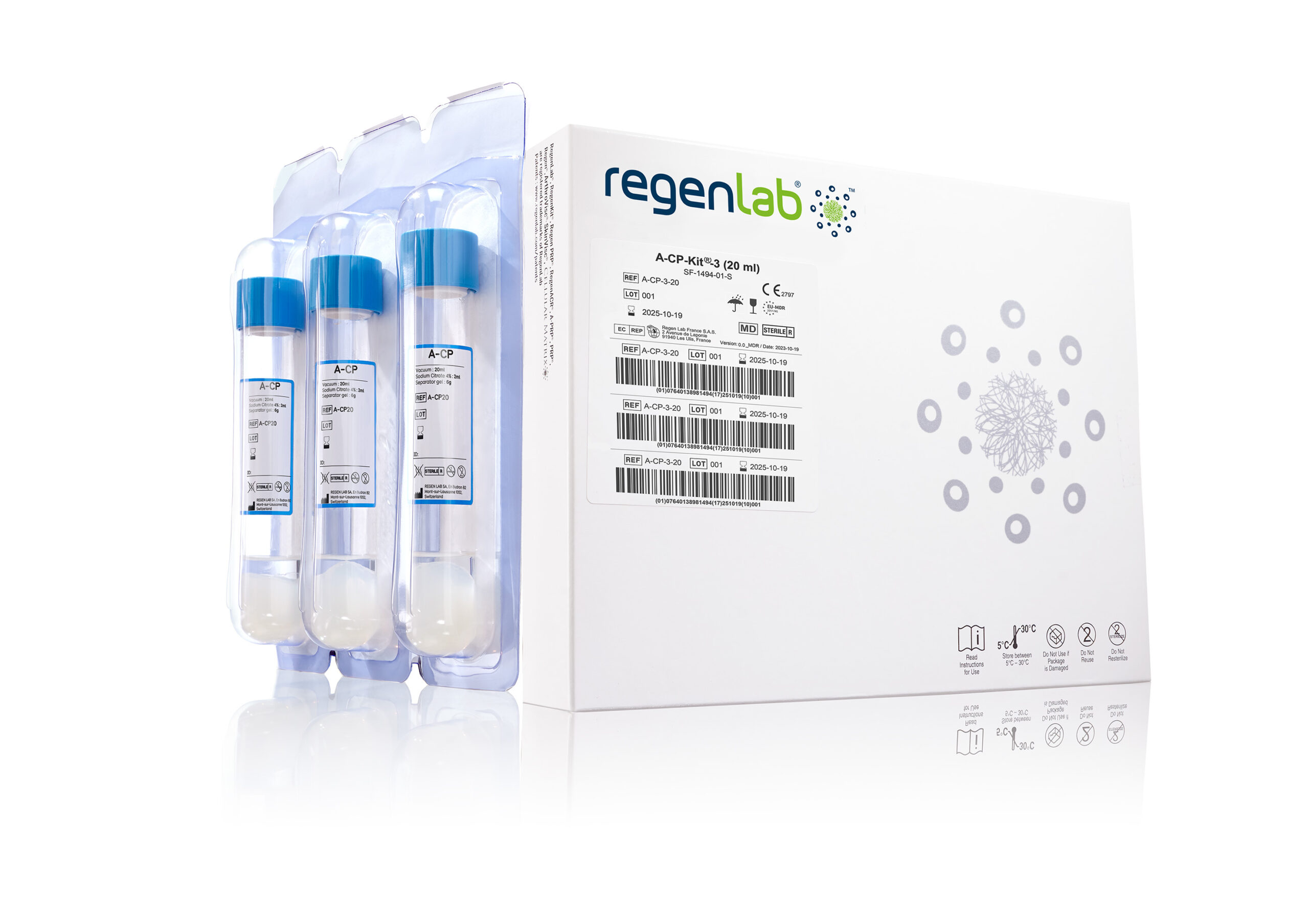
A-CP kit® 20mL) for preparing RegenPRP™.
REGENKIT-BCT® AND A-CP-KIT® ARE TWO DEVICES INNOVATIVE MEDICAL PRODUCTS FROM REGENLAB FOR PREPARATION OF REGENPRP, AN AUTOLOGOUS PRP OF STANDARDIZED COMPOSITION
RegenBCT®, available in 10 mL format (Table 1) and A-CP®, available in 20 mL, offer an effective solution for tissue regeneration (Figure 1).
These cutting-edge products reflect RegenLab’s ongoing commitment to excellence in healthcare and medical research.
RegenPRP™ glue in autologous skin grafting :
RegenPRP™ is a regenerative therapy that uses the healing properties of platelets and growth factors contained in blood plasma to stimulate tissue healing and regeneration.
Growth factors, such as EGF (epidermal growth factor), FGF (fibroblast growth factor), PDGF (platelet- derived growth factor), TGF-β (transforming growth factor beta), TGF-α (transforming growth factor alpha) and VEGF (vascular endothelial growth factor), are bioactive molecules that play a crucial role in the healing and tissue regeneration process.
When RegenPRP™ is activated by platelets, it releases these growth factors, promoting angiogenesis (the formation of new blood vessels), cell proliferation and differentiation, and the synthesis of new extracellular matrix.
This cascade of biological reactions helps accelerate the healing process and regenerate damaged tissue.

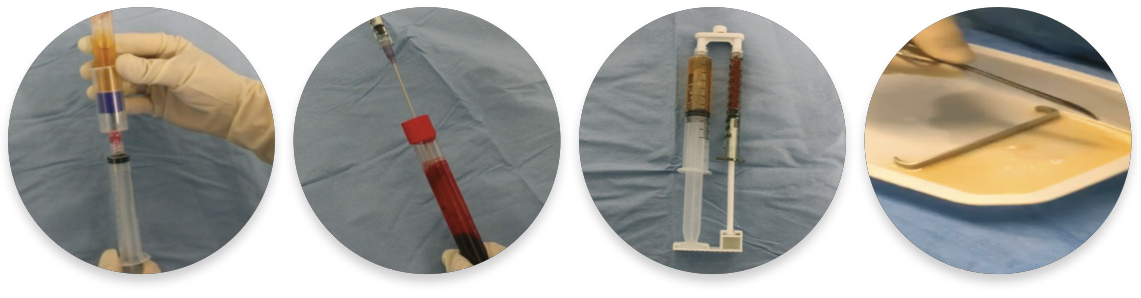
Figure 2. Simple, effective preparation of autologous RegenPRP™ in combination with activated thrombin serum (RegenATS®) and calcium gluconate, using RegenKit® Surgery.
FIBRIN GLUE ENRICHED WITH AUTOLOGOUS PLATELETS FOR SKIN GRAFTS
The tubes, manufactured by RegenLab, are vacuum tubes that contain an anticoagulant solution of sodium citrate and a chemically inert separator gel.
A small amount of the patient’s blood, 10 or 20 mL depending on the device, is drawn directly from the tube.
This blood is then centrifuged to separate the blood components, and, thanks to the specific density of the separator gel, isolate the platelets and plasma in the upper part of the tube.
Anticoagulation with sodium citrate has two advantages. It has no ancillary effect on the patient and is completely reversible.
It is therefore possible to induce coagulation and prepare RegenPRP in the form of platelet gel, autologous platelet-enriched fibrin glue, or fibrin clot.
To activate the coagulation of RegenPRP, autologous thrombin-rich serum (called ATS) is prepared from the patient’s blood with a RegenATS® tube.
This tube also allows the separation of blood components, but since it does not contain an anticoagulant, the plasma isolated above the separator gel coagulates.
Serum extracted from the coagulum contains all the enzymes in the coagulation cascade in their active form, including trombin which is the enzyme that converts soluble plasma fibrinogen into fibrin which polymerizes and forms the clot.
When RegenPRP is combined with TSA, these enzymes counteract the effect of sodium citrate and physiologically induce the coagulation of RegenPRP. (Figure 2 and Figure 3).
Autologous thrombin-activated RegenPRP, in the form of platelet gel or autologous fibrin glue, offers a promising approach to stimulating tissue healing and regeneration in a variety of clinical applications, ranging from wound healing to promoting tissue rejuvenation.
The aim of the 2017 study [1] was to determine whether RegenPRP in the form of autologous platelet-enriched fibrin glue could accelerate and improve wound healing after skin reconstruction in cases of tissue loss secondary to soft tissue infection.

Figure 3. RegenBCT® and RegenATS® tubes to obtain a combination of RegenPRP™ and activated thrombin serum.
INDICATIONS WITH SPLIT SKIN GRAFT RECONSTRUCTION FOR TISSUE LOSS SECONDARY TO NECROTIZING FASCIITIS OR FOURNIER’S GANGRENE
27 patients were randomized into two groups:
- Experimental treatment (14): Reconstruction with application of RegenPRP™ glue (Figure 4 and Figure 5),
- Conventional treatment (13): Reconstruction without RegenPRP™ glue
The mean time to complete healing was significantly shortened by almost 50% when autologous RegenPRP™/activated thrombin gel was combined with split-thickness skin graft compared with split- thickness skin graft alone (Figure 6).
In the study group, patients achieved a skin graft success rate of 89.6 ± 13.2%, whereas in the control group, graft success was 76.8 ± 11.5% (p = 0.01**) (Figure 7).
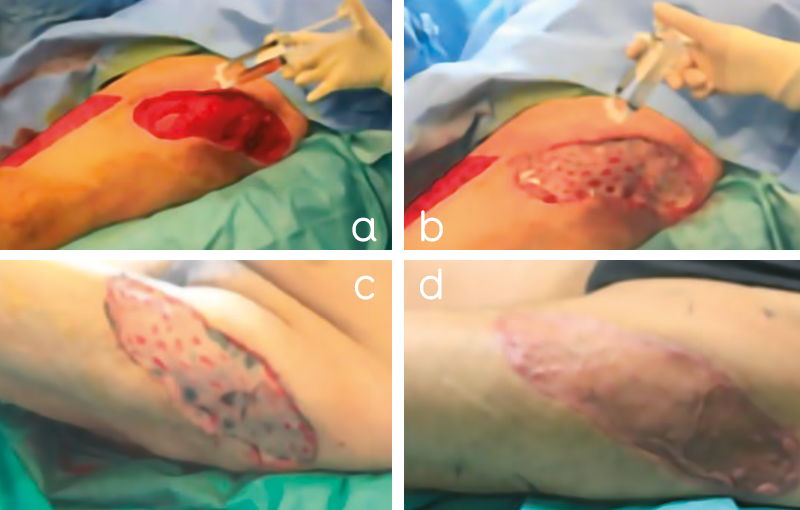
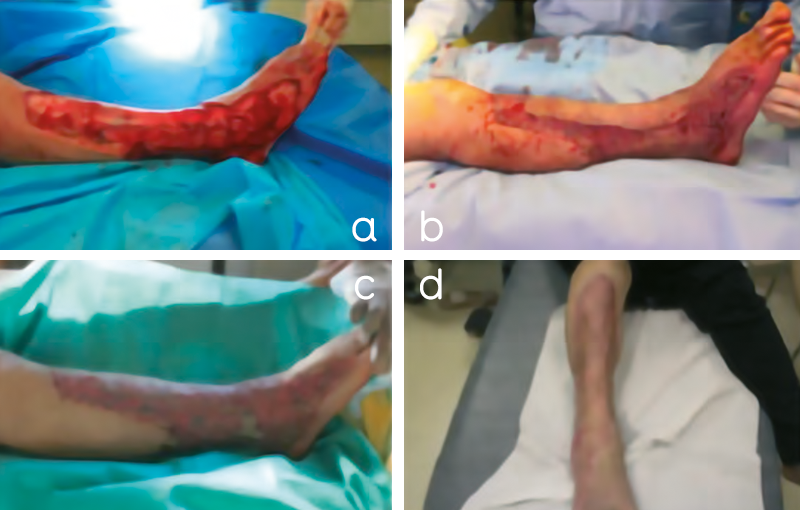
Figure 4. Patient 1, a 54-year-old man with extensive skin necrosis (2% of total body surface area) on the left thigh. A thorough and precise surgical debridement of all necrotic tissue was performed in the operating room. A. After wound bed preparation, A-PRP/thrombin gel was sprayed onto the wound bed and B. onto the split-thickness skin graft after fixation with staples. C. shows wound healing 2 days after skin grafting. D. Complete healing was achieved 21 days after skin grafting. The patient is discharged with mobilization possible 5 days after skin reconstruction.
Figure 5. Patient 2, a 44-year-old man with Fournier gangrene. Aggressive debridement of the necrotic tissue was performed in the operating room. A. After wound bed preparation, A-PRP/thrombin gel was sprayed onto the wound bed and B. onto the split-thickness skin graft after fixation with staples. C. The lower left figure shows wound healing 2 weeks after skin grafting. D. Complete healing was achieved 45 days after skin grafting
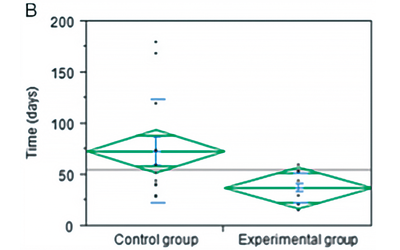
Figure 6. Percentage of skin grafts taken at 7 days.
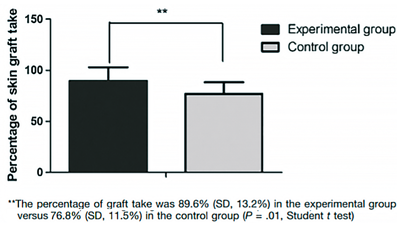
Figure 7. Percentage of skin grafts taken at 7 days.
RegenPRP™ GLUE IN WEIGHT LOSS SURGERY AND BREAST REDUCTION
The aim of this study [2] was to evaluate the adhesive action of autologous RegenPRP™ glue in plastic surgery and analyze its impact on post-operative complications.
- Modern plastic surgery is constantly seeking to improve clinical outcomes while minimizing risks to
- With this in mind, there is growing interest in the use of innovative products such as RegenPRP™ glue as a potential tool for optimizing healing and reducing post-operative complications (Figure 8).
Fifty-one patients who underwent breast reduction surgery, abdominoplasty, thigh lift (vertical and horizontal) or brachioplasty with application of RegenPRP glue to the entire surface of the subcutaneous tissue at the time of suturing were included in this study.
A retrospective control group operated by the same surgeons using the same techniques but without application of RegenPRP glue was used as a comparator.
RegenPRP™ glue reduced the incidence of seroma formation after limb lift surgery and prevented the occurrence of hematomas after breast reduction surgery (Table 2 and Table 3).
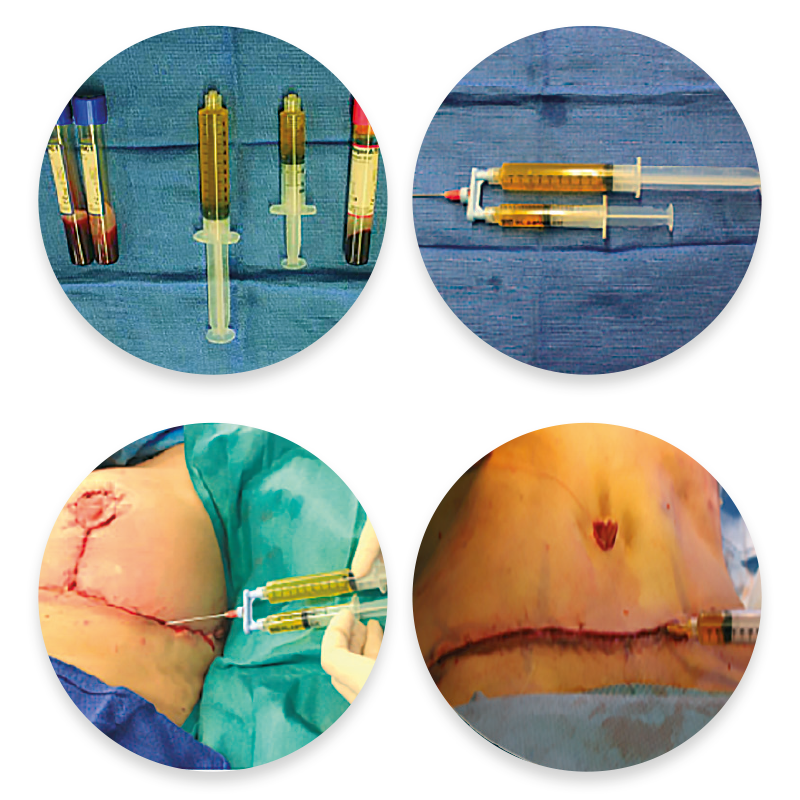
Figure 8. Preparation and application of RegenPRP™ glue from the RegenKit® Surgery, between the wound edges prior to suturing.

RegenPRP™ AS AN ADJUVANT FOR THE TREATMENT AND PREVENTION OF KELOID SCARS
After conventional treatments, keloid scars show varying degrees of recurrence.
The aim of this study [3] was to evaluate the efficacy and safety of RegenPRP™ in the treatment of postoperative keloid scars refractory to conventional treatments.
This prospective pilot study involved 17 patients with keloid scars who had failed to respond to 4 cortisone injections or radiotherapy after extra-lesional keloid resection.
RegenPRP™ was injected intraoperatively and then 3 times at 1-month intervals. The primary endpoint was complete remission of keloid scars 2 years after treatment. The severity of scar pruritus was recorded before and after treatment.
Nine keloid scars (53%) were completely resolved at 2 years, and 5 (29%) completely relapsed after treatment (Figure 9).
The pruritus severity score was significantly lower at 2 years than at baseline (1.33 ± 0.97 before treatment and 0.40 ± 0.63 at 2 years, P < 0.003) (Figure 10.
The mean score of the Vancouver Scar Scale was significantly improved (8.18 ± 2.38 before treatment and 3.82 ± 1.98 at 2 years, P < 0.001) (Figure 11).
RegenPRP™ injection is a safe and effective adjunctive treatment to resection for keloid scars refractory to conventional therapy.
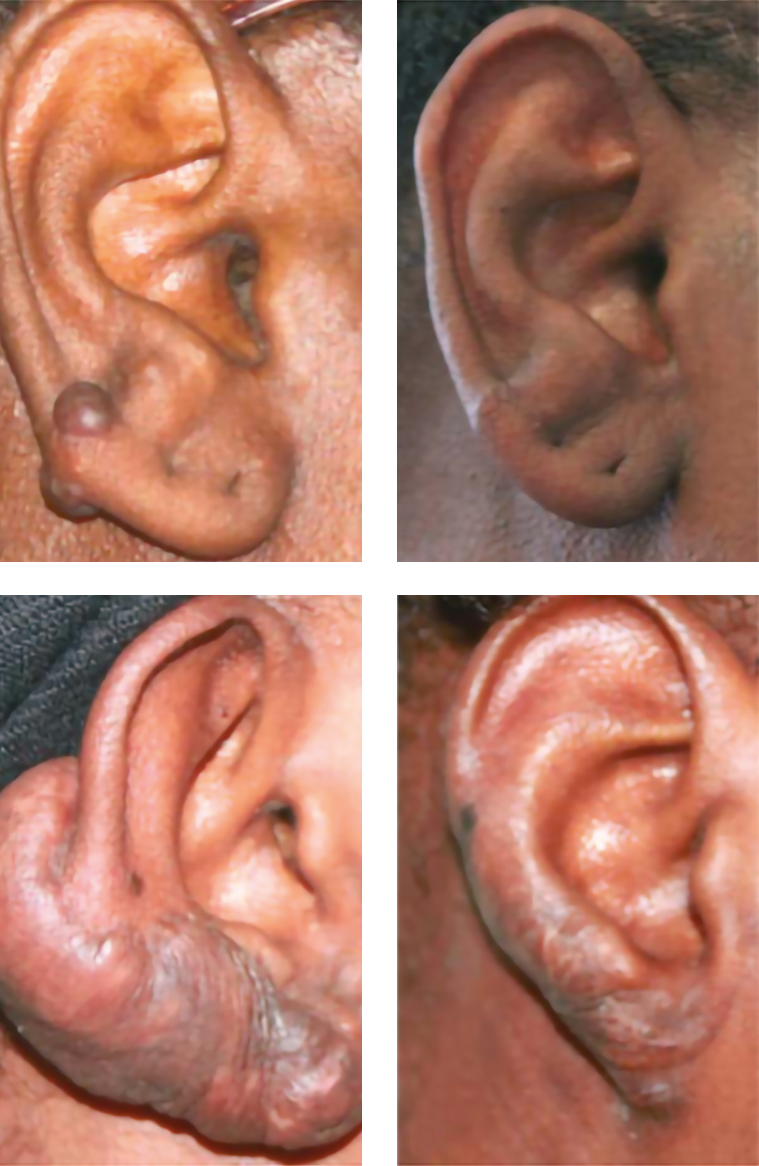
Figure 9. Two clinical cases of keloid scars in the earlobe that did not relapse (A) and appearance of the keloid scar before treatment (C); at 2 years (B and D), the scars did not relapse.
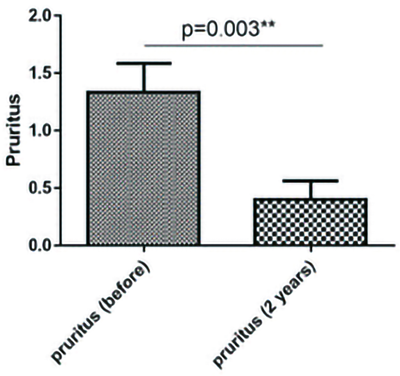
Figure 10. Significant reduction in pruritus severity 2 years after treatment
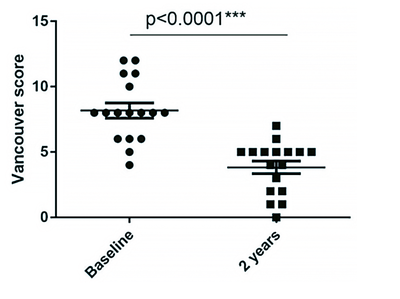
Figure 11. Significant reduction in VSS score showing improvement in scar quality 2 years after treatment.
CELLULAR MATRIX®: RegenPRP™ COMBINED WITH HYALURONIC ACID (HA) ON FACIAL SKIN REJUVENATION
The Cellular Matrix® kits are class III medical devices allowing the preparation of RegenPRP combined with hyaluronic acid (HA) in a closed circuit.
The aim of this study [4] was to evaluate the clinical benefit of combining RegenPRP™ and “Cellular Matrix®” HA in mesotherapy for skin rejuvenation (Figure 12).
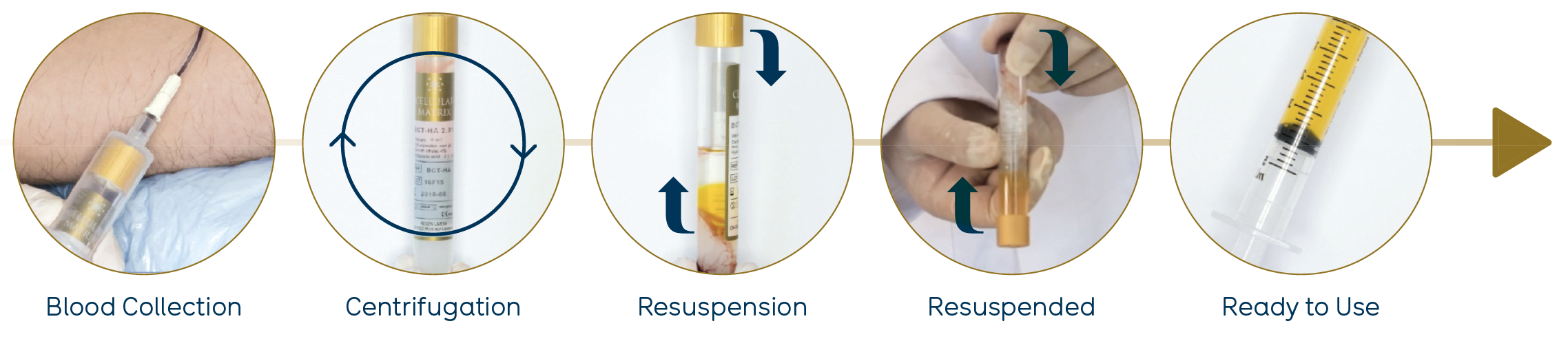
Figure 12. Preparation of RegenPRP™ combined with HA from the Cellular Matrix® BCT-HA Kit.
This was a prospective study involving 31 patients. The primary endpoint was measured by the efficacy of RegenPRP™ combined with HA in improving facial appearance, as assessed by the validated FACE-Q scale. ®The secondary endpoint was measured by the efficacy of RegenPRP™ combined with HA in improving skin texture «elasticity and firmness». Follow-up was carried out at 1, 3 and 6 months after 3 injection sessions (Figure 13 and Figure 14).
There was a significant improvement in FACE-Q scores after three treatment sessions and a significant improvement in skin elasticity parameters. All adverse events were reversible and well tolerated (Figure 15).
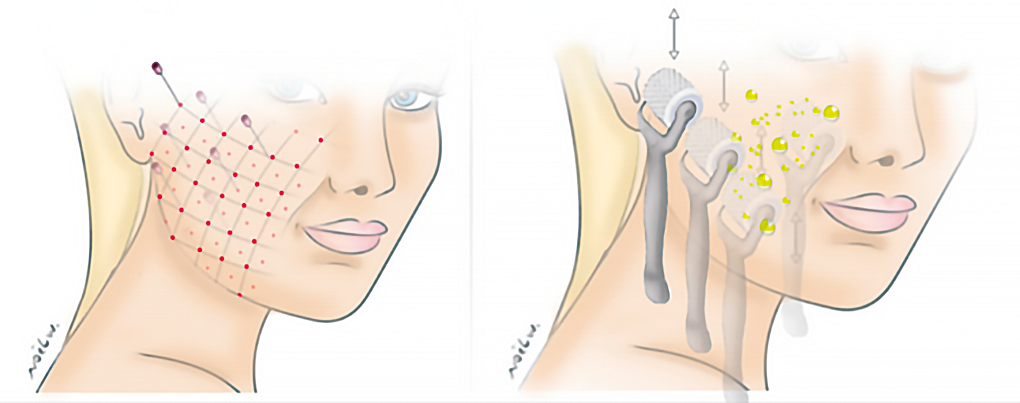
Figure 13. Illustration of Cellular Matrix® injection techniques (RegenPRP™ + hyaluronic acid).
Figure 14. Before and after of a patient after three RegenPRP™ injection sessions combined with HA.
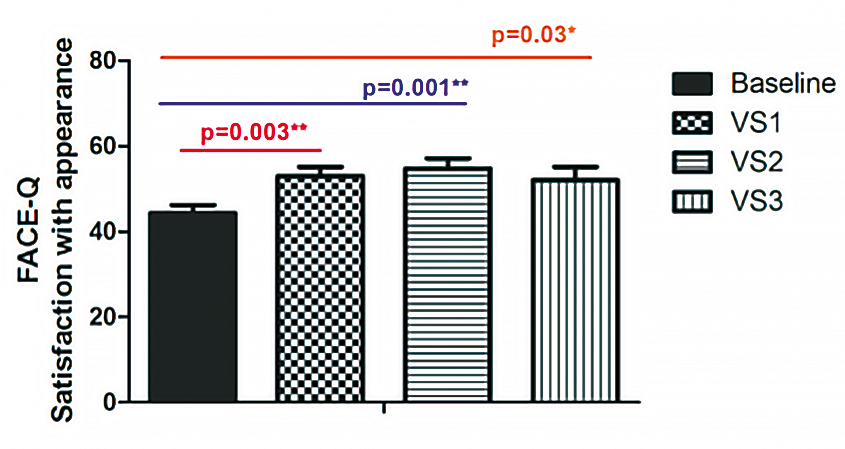
Figure 15. Evaluation of the FACE-Q appearance satisfaction scale.
INJECTION OF CELLULAR MATRIX® (RegenPRP™ COMBINED WITH HYALURONIC ACID) FOR THE TREATMENT OF VULVOVAGINAL DRYNESS IN POSTMENOPAUSAL WOMEN WITH A HISTORY OF BREAST CANCER
Approximately 50-70% of breast cancer survivors are affected by one or more symptoms of vulvovaginal atrophy (VVA).
For those unable to undergo hormone therapy, Cellular Matrix®: RegenPRP™ combined with hyaluronic acid (A-PRP-HA) (Figure 16) may offer a new alternative therapy for the treatment of VVA in postmenopausal women with a history of breast cancer.
We recruited 20 postmenopausal breast cancer survivors with VVA and a Gloria score of < 15.
The Bachman Vaginal Health Index (VHI) comprised five items, including: vaginal pH, elasticity, fluid volume (secretions), epithelial integrity and moisture.
We administered intramucosal injections of Cellular Matrix® (Figure 17) and performed clinical
assessments at 0, 1, 3 and 6 months.
The primary endpoint was assessment of vulvovaginal mucosal changes using the VHI. The secondary endpoint was the assessment of dyspareunia and sexual dysfunction on the basis of the Female Sexual Distress (FSD) score.
All participants (20 women) showed improvement in clinical symptoms of vaginal dryness and dyspareunia.
The VHI score showed a significant increase at 1, 3 and 6 months, rising from a baseline (pre-treatment) total score of 10.7 ± 2.12 to 20.75 ± 4.8 (P < 0.0001) at 6 months.
An improvement in vaginal hydration and epithelial integrity was reported.
Figure 16. Platelet-Rich Plasma combined with a hyaluronic acid preparation (PRP-HA) with Cellular Matrix® /BCT-HA. After centrifugation, the blood is fractionated: red blood cells are trapped under the gel, and cellular elements are deposited on the gel surface. Hyaluronic acid is placed on top of the PRP. Gently invert the BCT-HA tube several times to resuspend the cell deposit in the supernatant and homogenize the hyaluronic acid with the PRP. Approximately 4 mL of PRP-HA mixture will be obtained for each tube. BCT-HA, blood cell therapy-hyaluronic acid.
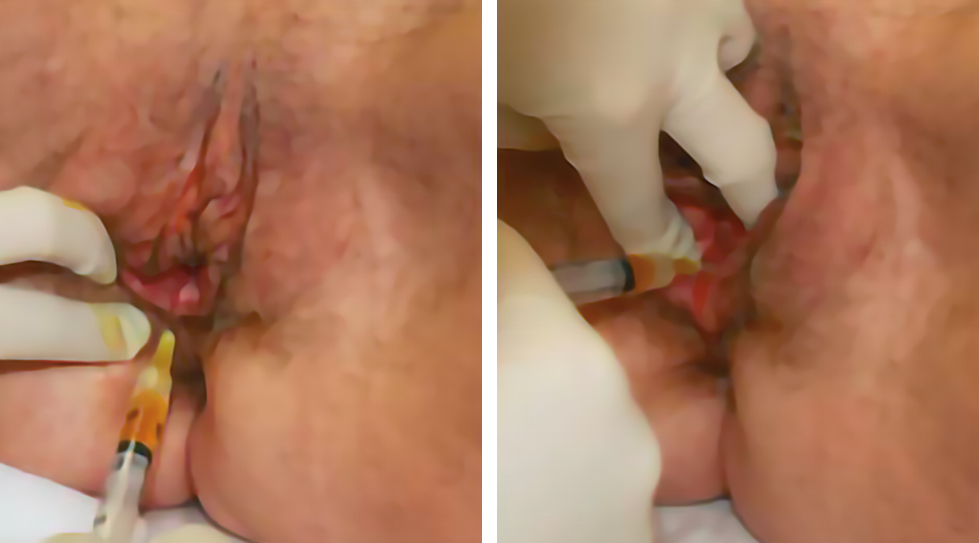
Figure 17. A 56-year-old woman with episiotomy sequelae. Mucosal and submucosal injections of 4 mL of PRP-HA with a 27G needle into the vaginal wall (posterior) and vestibule (under scars and fissure). Cellular Matrix® (RegenPRP™ combined with hyaluronic acid).
A VHI score of > 15 showed a positive therapeutic outcome. The pH showed a significant decrease (Figure 18).
The FSD score decreased significantly over the course of the study, from an initial score of 36.35 ± 2.53 before treatment to 30.15 ± 2.47 6 months after treatment, representing an improvement of 17% (P < 0.0001, respectively). No adverse events were reported (Figure 19).
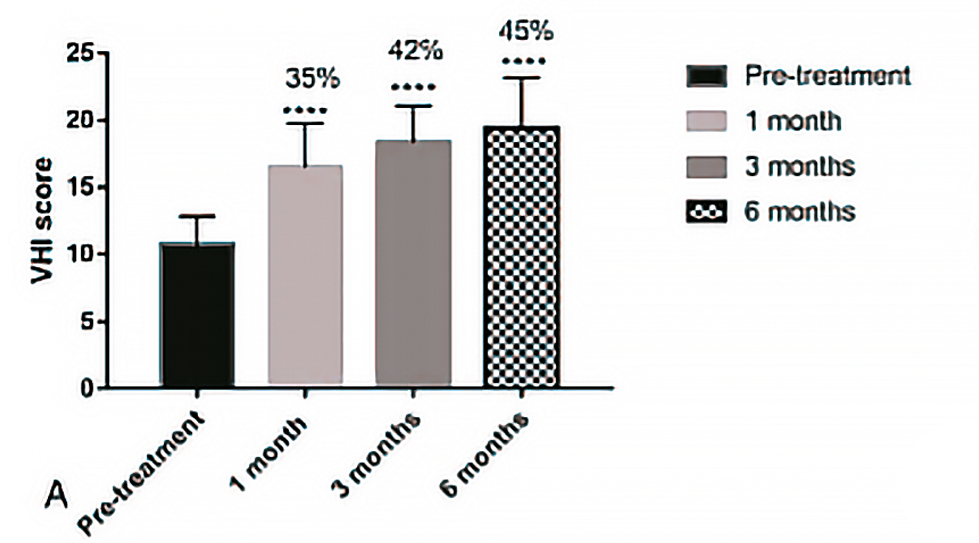
Figure 18. A. Time course of mean pH from baseline (pre-treatment) to 1 to 3- and 6-months post-treatment. Bars represent standard deviation. P<0,0001.
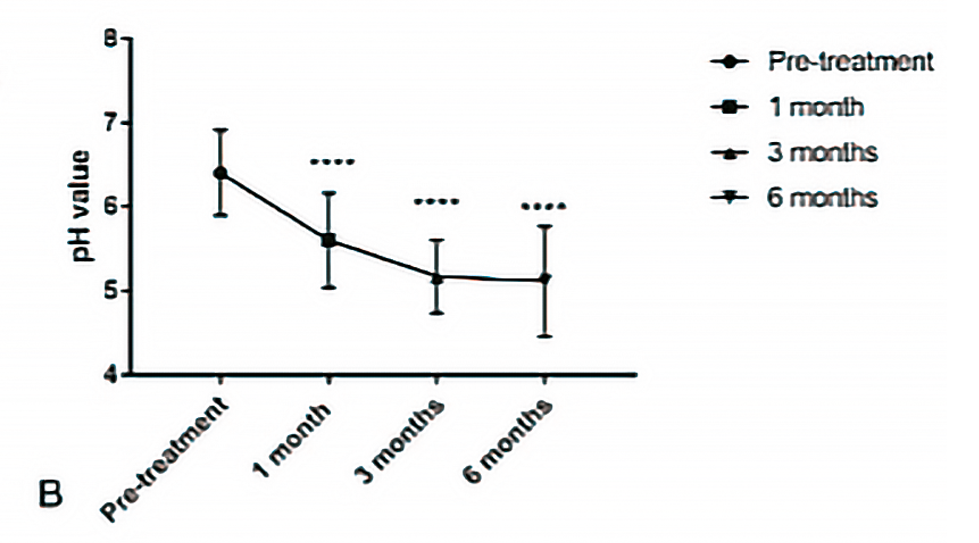
Figure 18. B. Mean change in VHI and percentage improvement from baseline (pre-treatment) to 1 to 3- and 6-months post-treatment. Results represent mean standard deviation (SD). P<0,0001. VHI, Vaginal Health Index.

Figure 19. Female Sexual Distress (FSD) and percentage improvement from baseline (pre-treatment) at 1, 3 and 6 months post-treatment. Results represent mean standard deviation (SD). P<0.04, P<0.0001
EFFECT OF PRP ON POTENTIATION OF MESENCHYMAL STEM CELLS IN SKIN HEALING
Chronic and acute non-healing wounds represent a major public health problem, and replacing skin lesions with newly regenerated skin is a challenge.
Mesenchymal stem cells (MSCs) and platelet-rich plasma (PRP) have been tested separately in attempts to regenerate lost skin.
However, these treatments were often ineffective in achieving complete wound healing.
Further studies suggest that PRP could be used in combination with MSCs to enhance the efficacy of cell therapy on tissue repair. However, systematic studies related to the effects of PRP on MSC properties and their ability to rebuild the skin barrier are lacking.
The model used [6] was a 6-week-old immunocompetent male mouse (C57BL/6JRj) with 4 full- thickness wounds (Figure 20).
We evaluated the skin repair capacity of a treatment combining human adipose MSCs and human PRP compared with treatment with saline, PRP alone or MSCs alone.
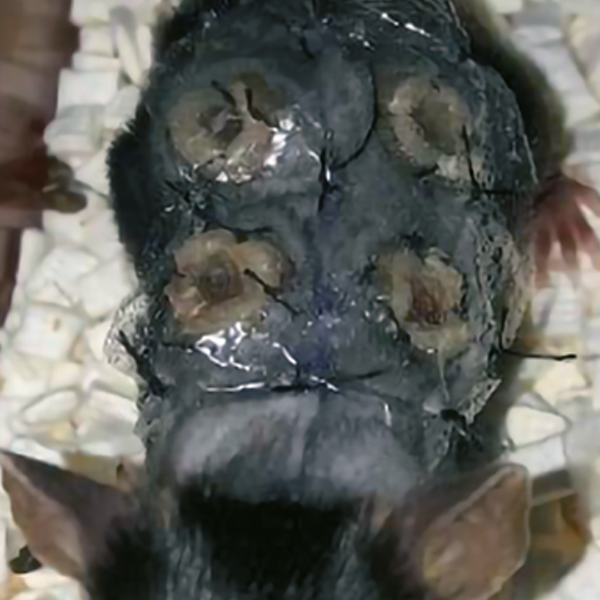
Figure 20. A. Experimental model of 6-week-old, immunocompetent male C57BL/6JRj mice.
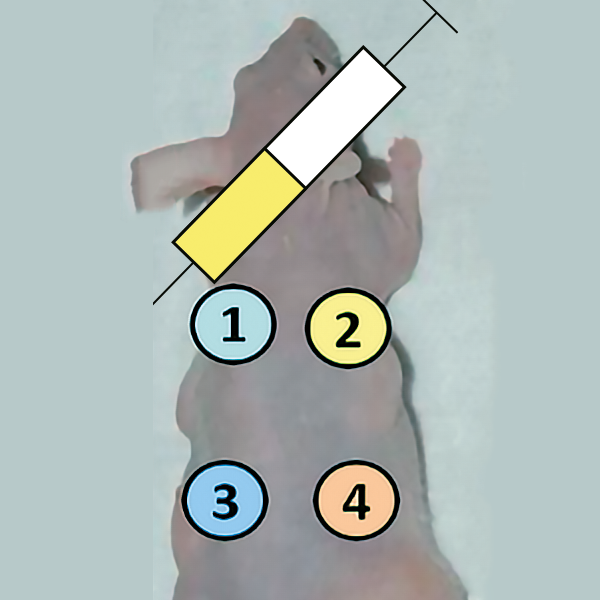
Figure 20. B. Experimental scheme – zone 1: injection of MSC, zone 2: injection of PRP 20% + CaCl 10%, zone 3: MSC, zone 4: injection of PRP 20% + CaCl 10% + MSC.
Macroscopic analysis was performed to measure the percentage of skin regeneration for the different conditions and at different times (D3-J7).
The percentage of skin regeneration for control (CLT), PRP alone, CSM alone and CSM combined with PRP conditions at D3.
Results showed that PRP alone significantly increased skin regeneration, from 8% in the control condition to 23% in the PRP group (p < 0.001).
MSC alone and MSC+PRP significantly increased skin regeneration compared with control p<0.0001. The percentage of skin regeneration increased from 8% for CLT to 32% for MSC alone and 45% for MSC+PRP.
At D7, the combination of MSCs and platelet concentrates significantly increased skin regeneration compared with the control group (p < 0.001).
Skin regeneration rose from 43% in the control group to 80% in the MSC+PRP group. A significant increase was also observed in the MSC+PRP condition compared with the MSC alone condition (p < 0.01) and the MSC alone condition compared with the control (p < 0.05) (Figure 22A and B).
At D10, no significant difference was observed between the different conditions.
Comparison of total healing times, corresponding to total epidermalization of the lesion (complete closure), shows that the MSC+PRP combination significantly reduces total healing time compared with the control (P < 0.01) (Figure 22C).
An analysis of skin biomechanical parameters was carried out after complete healing at D15 in order to compare skin elasticity in the different conditions. The results of the coarse elasticity measurement show a significant improvement (R2) in the PRP, CSM and CSM+PRP conditions compared with the control group. There is a greater improvement in the R2 parameter in the CSM+PRP condition compared with the other conditions (Figure 21).
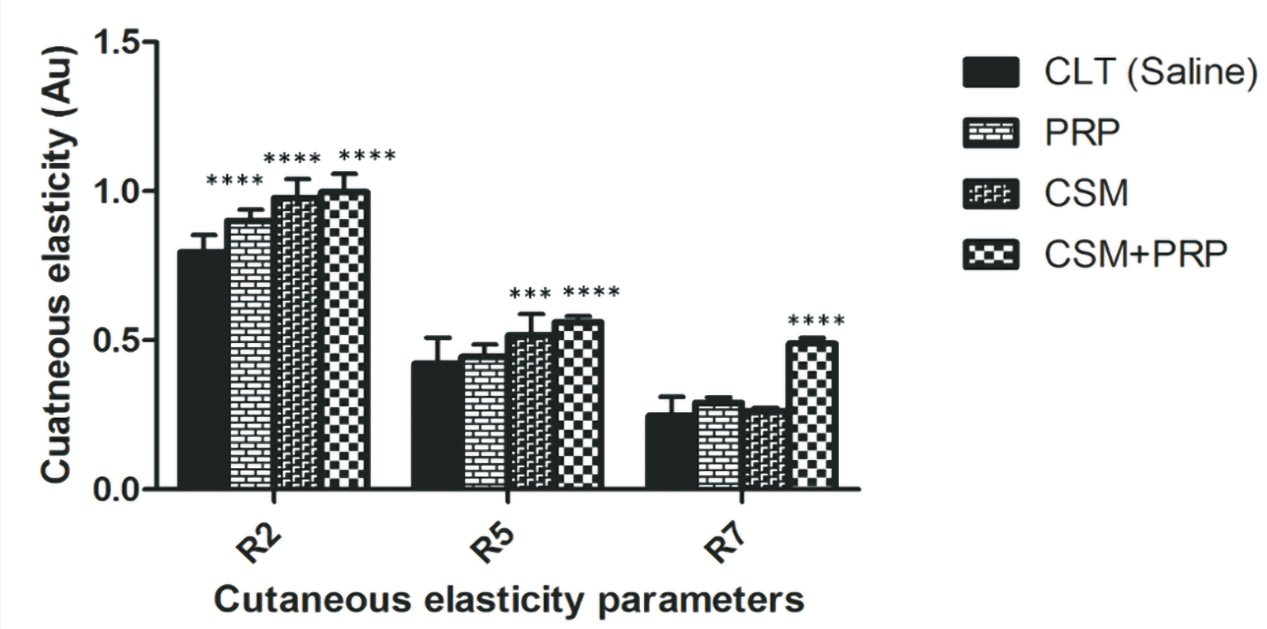
Figure 21. Biomechanical parameters R2 (gross elasticity), R5 (net elasticity), R6 (viscoelasticity ratio), and R7 (biological elasticity) of the mouse healed wound following their treatment with either saline solution, PRP, hMADS cells, or hMADS cells in combination with PRP, respectively. (a-d) Results represent the mean ± SD obtained from n = 10 mice. ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001
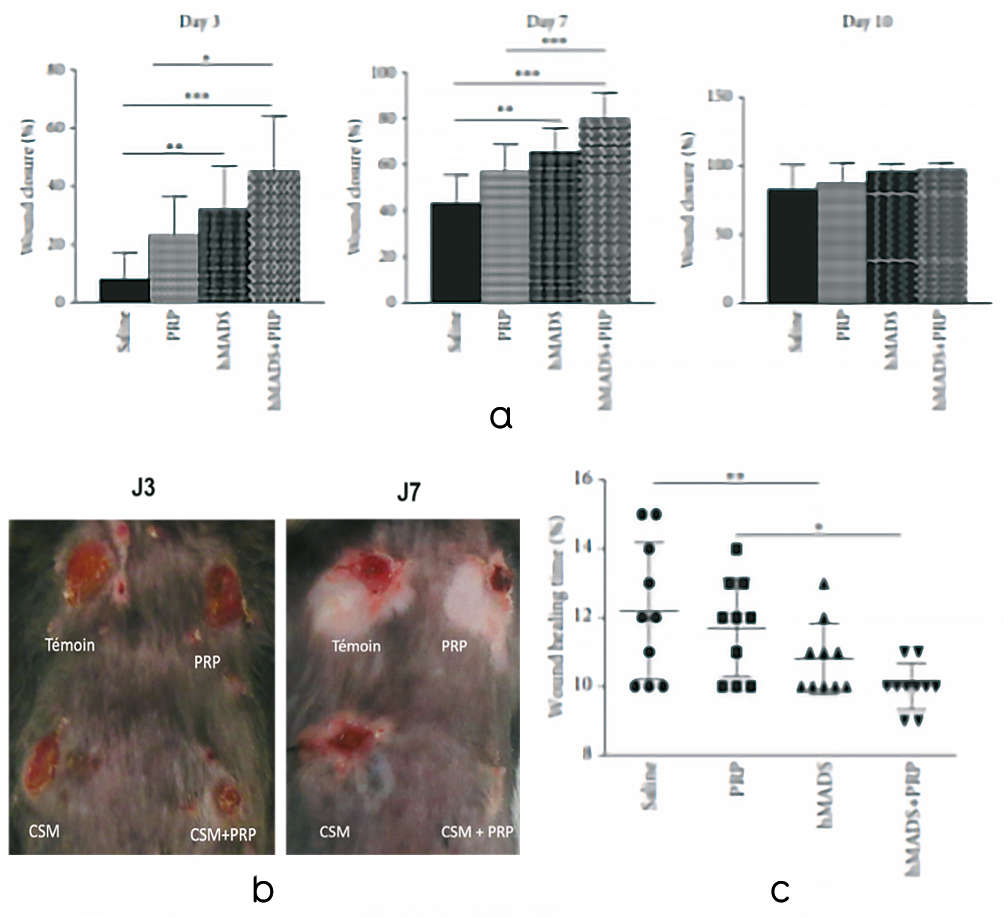
Figure 22. PRP treatment improves the healing potential of human MSCs (hMADS cells) following their grafting into mouse wounds. A. Closure rates of wounds treated with saline, PRP, hMADS cells or hMADS cells in combination with PRP at 3-, 7- and 10-days following surgery. B. Representative photograph of the back of a mouse with skin wounds at 3- and 7-days following wounding and treatment with saline, PRP, hMADS cells or hMADS cells in combination with PRP, respectively. C. Wound healing times of mice treated with saline, PRP, hMADS cells or hMADS cells in combination with PRP, respectively. *p < 0.05, **p < 0.01, ***p < 0.001.
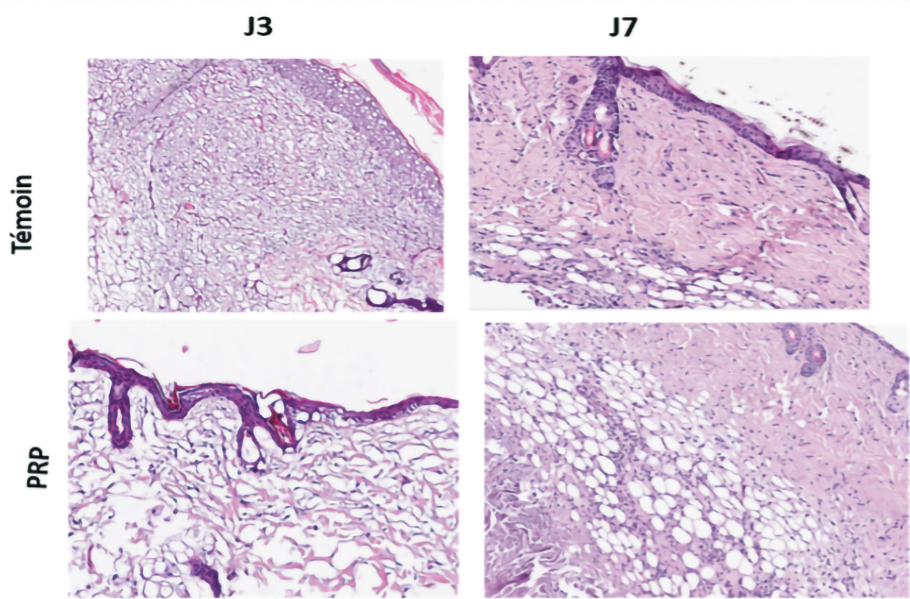
Figure 23. Hematoxylin-eosin staining of the dermis.
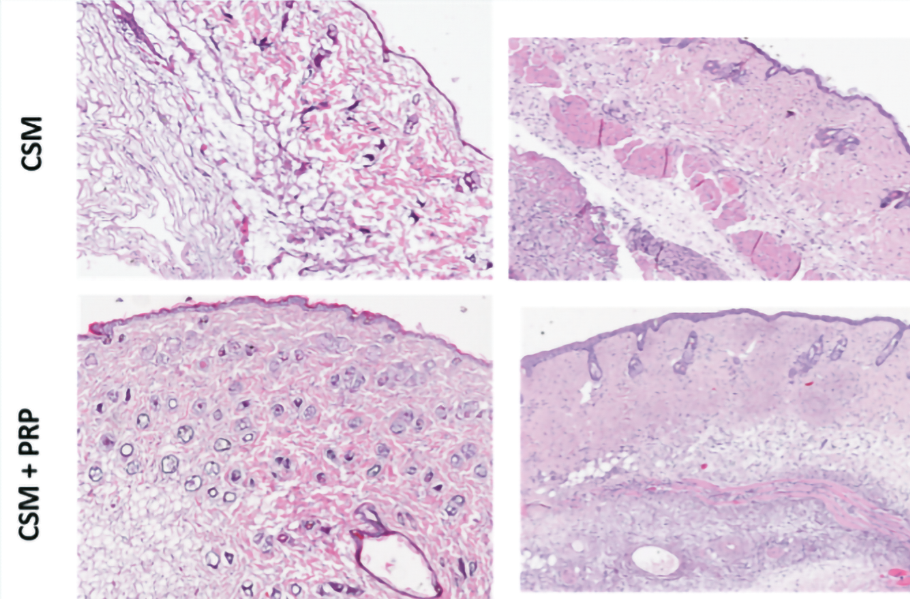
Histology showed in the MSC condition in combination with PRP, a better organization of the dermis with more collagen fibers and myofibrils and a greater number of capillaries reflecting neo angiogenesis.
With regard to epidermal and dermal thickness, the analysis showed no qualitative difference (Figure 23).
Capillary density of biopsies at D3 and D7 after hMADs grafting was determined after microvessel labeling with B4 isolectin B4.
Immunohistochemistry showed a highly significant difference in capillary density for MSC grafts alone and MSC+PRP (p < 0.001***) at D3.
A significant difference was observed between lesions treated with MSCs alone and those treated with PRP at D3 (p < 0.05*).
At D3, MSC+PRP significantly increased capillary density and thus angiogenesis compared with the MSC alone group (p < 0.05*) and lesions treated with PRP alone (p < 0.0001****).
At D7, a significant increase in capillary density was observed only for MSC+PRP lesions compared with the control (HBSS treatment) (p < 0.05*) (Figure 24).
In order to demonstrate the activation of the MSC secretome in the presence of PRP, we analyzed the transcriptional expression of VEGF, HGF and SDF-1 genes by quantitative RT PCR from biopsies of MSCs grafted naïve or in the presence of 20% PRP, obtained after sacrifice of the mice on D1 and D3.
The results summarized in the figure show significant activation of the MSC secretome in the presence of 20% PRP compared with naive cells, with a significant increase in the transcriptional expression of the 3 genes analyzed, VEGF, HGF and SDF-1 at D1 and D3.
The pro-angiogenic factors VEGF and HGF were significantly increased at D1 (p < 0.0001****, p < 0.001*** respectively) and D3 (p < 0.001***, p < 0.01** respectively) in biopsies treated with MSC+PRP compared with MSC alone.
In parallel, the chemoattractant factor SDF-1 was significantly increased at D1 (p < 0.01*) and D3 (p < 0.01*) in biopsies treated with MSC+PRP compared with MSC alone.
These results confirm those obtained in vitro, showing that PRP increases the pro-angiogenic and chemoattractant capacity of MSCs (Figure 25).
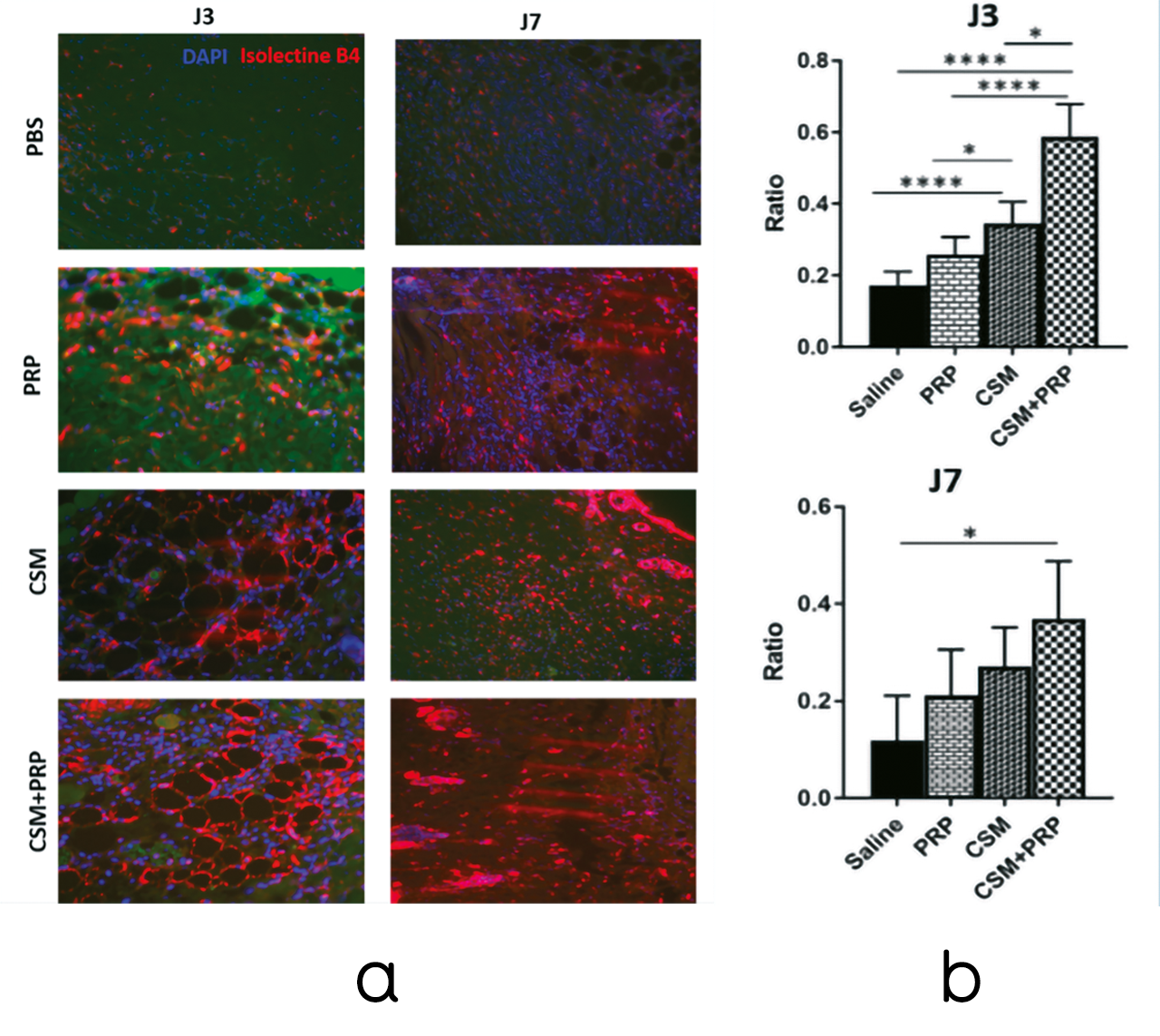
Figure 24. PRP treatment stimulates the pro-angiogenic properties of grafted human MSCs (hMADS cells) in mouse wounds. A. Representative isolectin B4 immunostaining of epithelial cells (red signal) 3 days after treatment with saline, PRP, hMADS cells or hMADS cells in combination with PRP, respectively. Nuclei were counterstained with DAPI (blue signal). Scale bar, 50 μm. B. Quantification of B4-positive isolectin cells in mouse wounds at 3 and 7 days after treatment with saline, PRP, hMADS cells, or hMADS cells in combination with PRP, respectively. ∗p < 0.05, ∗∗∗∗p < 0.0001.
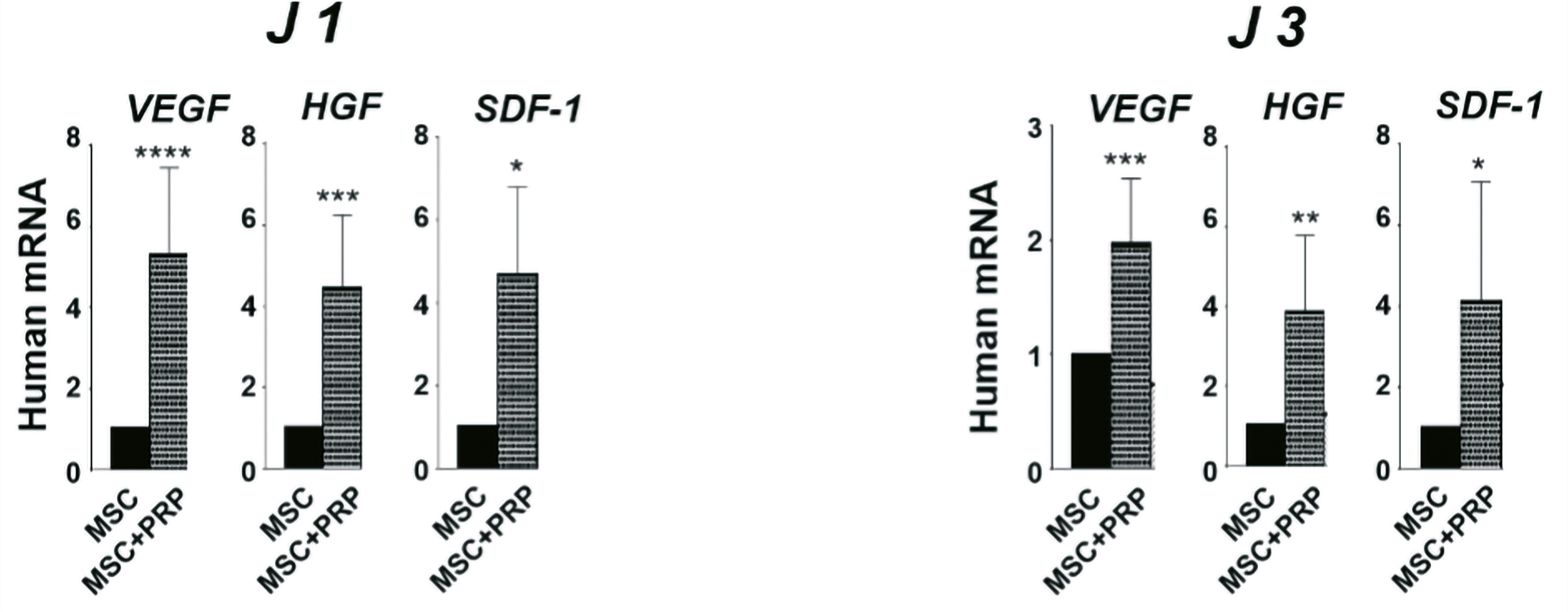
Figure 25. Relative transcriptional expression of human VEGF and human SDF-1 in mouse wounds corresponding to expression in hMADS cells grafted in combination with PRP compared with expression in hMADS cells grafted alone, at 1-, 3- and 7-days post-wounding. Results represent mean ± SD obtained from at least n = 5 mice per group. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001.
RESEARCH PROJECT: A PROSPECTIVE PILOT STUDY OF THE NASOLABIAL FOLD
The Regen Matrix® medical device (under development, not yet certified) is an extension of the Cellular Matrix® device.
When PRP and XLHA are used in combination, the desired effects are enhanced and prolonged. XLHA creates an optimal “structural matrix” in which platelets gradually release their growth factors.
The tube contains 2 mL of cross-linked HA at a concentration of 20 mg/mL (40 mg in total), produced by bacterial fermentation, without animal proteins.
The Regen Matrix® device combines the benefits of cross-linked hyaluronic acid (XLHA) with those of platelet-rich plasma (RegenPRP™) in a single, closed-loop injection.
REFERENCES
- Hersant, B., et al. Autologous Platelet-Rich Plasma/Thrombin Gel Combined with Split-Thickness Skin Graft to Manage Postinfectious Skin Defects : A Randomized Controlled Study. Adv Skin Wound Care 2017 ; 30(11) : 502-8.
- Hersant, , et al. Efficacy of Autologous Platelet-rich Plasma Glue in Weight Loss Sequelae Surgery and Breast Reduction: A Prospective Study. Plast Reconstr Surg Glob Open 2016 ; 4(11) : e871.
- Hersant, , et al. Efficacy of Autologous Platelet Concentrates as Adjuvant Therapy to Surgical Excision in the Treatment of Keloid Scars Refractory to Conventional Treatments: A Pilot Prospective Study. Ann Plast Surg 2018 ; 81(2) : p. 170-5.
- Hersant, , et al. Efficacy of autologous platelet-rich plasma combined with hyaluronic acid on skin facial rejuvenation: A prospective study. J Am Acad Dermatol 2017 ; 77(3) : 584-6.
- Hersant, , et al. Efficacy of injecting platelet concentrate combined with hyaluronic acid for the treatment of vulvovaginal atrophy in postmenopausal women with history of breast cancer: a phase 2 pilot study. Menopause 2018 ; 25(10) : 1124- 30.
- Hersant, B., et al. Platelet-Rich Plasma Improves the Wound Healing Potential of Mesenchymal Stem Cells through Paracrine and Metabolism Alterations. Stem Cells Int 2019 ; 2019 : 1234-63.
